Car batteries, especially lead-acid batteries, are essential for vehicles to start and power electrical systems. But how are they made, and is the production process safe for humans and the environment? Let’s break down the process and explore the materials involved.
1. Basic Components of a Car Battery
A typical car battery consists of:
- Lead Plates: These plates store and release energy.
- Electrolyte Solution: A mixture of sulfuric acid and water that allows electricity to flow.
- Plastic Casing: Protects the internal parts and holds everything together.
- Terminals: Connects the battery to the car’s electrical system.
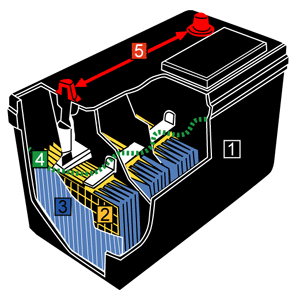
2. The Raw Materials: Metals and Chemicals
- Lead (Pb): Lead is the primary material in a car battery. Both pure lead and lead dioxide are used in the plates.
- Sulfuric Acid (H₂SO₄): This is a corrosive liquid used as the electrolyte to create a chemical reaction.
- Plastic (Polypropylene): The casing of the battery is made of durable plastic.
- Other Metals: Depending on the design, small amounts of other metals may be used in the terminals and connections.
Is the Production of a Car Battery Safe?
The production process can pose risks to both workers and the environment. Here’s why:
- Lead Exposure:
- Lead is toxic, especially in high amounts. Workers involved in handling and refining lead are at risk of lead poisoning if proper safety measures are not followed. Prolonged exposure can damage the nervous system, kidneys, and other vital organs.
- Lead fumes and dust, if inhaled or ingested, can be harmful. That’s why battery factories must have strict safety protocols to protect workers, such as wearing personal protective equipment (PPE) like masks and gloves.
- Sulfuric Acid:
- Sulfuric acid is highly corrosive. Direct contact can cause burns on the skin, and inhalation of fumes can damage the respiratory system.
- In factories, sulfuric acid must be handled with extreme care, and workers are required to wear protective gear to avoid burns and inhalation of fumes.
- Environmental Concerns:
- Lead mining and refining for battery production can lead to pollution if not managed properly. Lead-contaminated water and soil are significant environmental hazards.
- Battery production plants must have waste management systems in place to prevent contamination of local ecosystems.
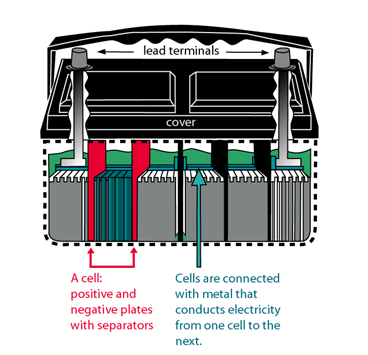
3. Is Car Battery Production Difficult?
While the process of assembling a car battery might seem simple, dealing with hazardous materials like lead and sulfuric acid requires specialized equipment and trained personnel. Some of the challenges include:
- Handling Toxic Materials: Lead and sulfuric acid are dangerous and must be handled carefully.
- Precision Manufacturing: The plates inside the battery need to be carefully aligned, and any imbalance can affect battery performance.
- Sealing and Charging: After assembly, the battery must be sealed to prevent leaks and charged to ensure it holds power.
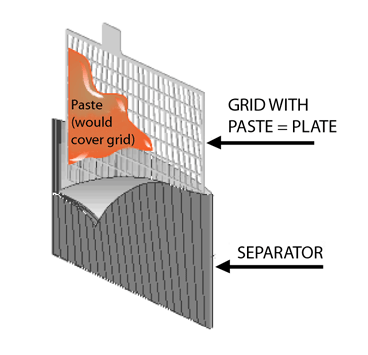
4. Safety Measures in Battery Production
To make the process safer for workers, manufacturers use several precautions:
- Ventilation Systems: Factories use high-grade ventilation systems to remove harmful lead fumes and acid vapors.
- PPE (Personal Protective Equipment): Workers must wear safety gear such as gloves, protective suits, and masks to limit their exposure to harmful chemicals.
- Recycling Programs: Since lead-acid batteries can be recycled, many manufacturers promote recycling to reduce environmental damage. In fact, up to 99% of the lead in old car batteries can be reused in new batteries.
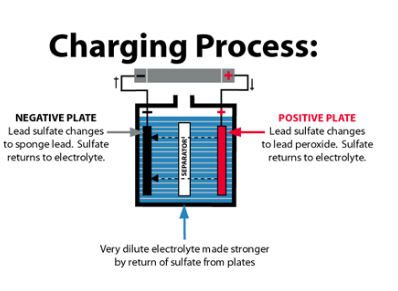
5. Are Batteries Safe for Humans?
Once produced, car batteries are generally safe to handle, as long as they are used properly. However, if the battery is damaged or leaks, the acid can cause skin burns, and lead exposure can be harmful. That’s why it’s important to:
- Handle batteries with care.
- Wear gloves when installing or removing them.
- Dispose of old batteries through recycling programs to prevent environmental contamination.

6. Sustainable Alternatives?
Newer technologies, like lithium-ion batteries, used in electric vehicles (EVs), are designed to reduce the environmental impact compared to traditional lead-acid batteries. However, they come with their own set of challenges, such as the mining of lithium and cobalt, which also raises safety and ethical concerns.
Conclusion
While car batteries are essential for vehicles, the production process involves handling hazardous materials like lead and sulfuric acid. This can pose risks to both workers and the environment if proper safety precautions aren’t taken. However, with strict regulations and recycling initiatives, many of these risks can be minimized. Lead-acid batteries can be recycled, making them one of the most recyclable products in the world, but handling them safely is crucial for both human health and environmental protection.





